Retort packaging plays a crucial role in preserving food for extended periods, making it a vital component in the food industry. The process involves heat sterilization to achieve commercial sterility, ensuring the food remains safe for consumption.
The debate between retort pouches and cans continues, with each having its advantages. Retort pouches offer flexibility and convenience, while cans provide durability and a long shelf life. The choice between the two often depends on the specific application and the type of food being packaged.
The materials used in retort packaging and the integrity of the seal are critical factors that determine the overall safety and effectiveness of the packaging. Ensuring the seal is intact prevents contamination and maintains the sterility of the food inside.
Key Takeaways
- Retort packaging is essential for preserving food through heat sterilization.
- The choice between retort pouches and cans depends on the application and food type.
- Material selection and seal integrity are critical for packaging safety.
- Commercial sterility is achieved through the retort process.
- Shelf-stable food packaging relies on effective retort packaging.
What Is Retort Packaging and Why It Matters for Food Preservation
Retort packaging is a critical component in the food industry, enabling the production of shelf-stable products through high-temperature sterilization. This process is crucial for ensuring the safety and quality of food products. By understanding retort packaging, manufacturers can better appreciate its role in achieving commercial sterility.
Retort Packaging Definition
Retort packaging refers to the process of sterilizing food products in a sealed container, typically using high-temperature steam or water, to achieve commercial sterility. This method is widely used for packaging low-acid foods, such as soups, meats, and vegetables, which require a high level of sterilization to prevent spoilage. The retort process involves heating the packaged food to a high temperature, usually above 212°F (100°C), to kill off bacteria and other microorganisms.
How Retort Packaging Achieves Commercial Sterility
Commercial sterility is achieved through the retort process by ensuring that the food product is heated to a sufficient temperature for a sufficient amount of time to kill off pathogenic microorganisms. This is typically done using a combination of heat, pressure, and time. The retort process is designed to ensure that the food is heated evenly throughout, preventing any underprocessed areas that could harbor bacteria. By achieving commercial sterility, retort packaging ensures that the food remains safe for consumption over an extended period.
The Role of High-Temperature Sterilization in Shelf-Stable Foods
High-temperature sterilization is a critical component of retort packaging, as it enables the production of shelf-stable foods. By sterilizing the food at high temperatures, manufacturers can ensure that the product remains safe for consumption without the need for refrigeration. This is particularly important for low-acid foods, which are more susceptible to spoilage. The use of high-temperature sterilization in retort packaging has revolutionized the food industry, enabling the mass production of shelf-stable products.
The following table summarizes the key aspects of retort packaging and its role in achieving commercial sterility:
| Aspect | Description | Importance |
|---|---|---|
| Retort Packaging Definition | Process of sterilizing food in a sealed container | Ensures commercial sterility |
| Commercial Sterility | Achieved through high-temperature sterilization | Ensures food safety |
| High-Temperature Sterilization | Enables production of shelf-stable foods | Critical for low-acid foods |
By understanding the principles of retort packaging and its role in achieving commercial sterility, manufacturers can produce high-quality, shelf-stable food products that meet consumer demands. The use of retort packaging has become a cornerstone of the food industry, enabling the mass production of safe and nutritious food products.
The Science Behind Retort Packaging Technology
Retort packaging technology relies on a complex interplay of heat, pressure, and material science to achieve commercial sterility in food products. This technology is fundamental to preserving food quality and ensuring safety.
Heat Sterilization Process Fundamentals
Heat sterilization is the cornerstone of retort packaging. It involves subjecting packaged food to high temperatures, typically between 212°F (100°C) and 275°F (135°C), to kill harmful bacteria and other microorganisms. The process is designed to achieve commercial sterility, which means the food is safe for consumption and has a long shelf life without refrigeration.
The effectiveness of heat sterilization depends on several factors, including the temperature applied, the duration of the heat treatment, and the type of packaging material used. Retort packaging materials are designed to withstand these high temperatures and pressures, ensuring that the packaging remains intact and the food inside is properly sterilized.
Achieving Commercial Sterility in Food Products
Achieving commercial sterility is critical for ensuring that food products are safe for consumption over an extended period. This involves not just killing harmful bacteria but also inactivating enzymes that could cause spoilage. The retort process is carefully controlled to achieve the required level of sterility while preserving the nutritional value and flavor of the food.
- Temperature control is crucial for achieving the desired level of sterility.
- The duration of the retort process affects the final product quality.
- Packaging material properties play a significant role in maintaining sterility.
How Retort Packaging Preserves Nutrition and Flavor
Retort packaging is designed not only to sterilize food but also to preserve its nutritional value and flavor. The high-temperature processing can affect the texture and taste of food, but modern retort packaging techniques minimize these effects. For instance, the use of flexible packaging like retort pouches allows for more efficient heat transfer, reducing the processing time and helping preserve the food’s natural qualities.
The science behind retort packaging technology is a testament to the advancements in food preservation. By understanding the principles of heat sterilization and material science, manufacturers can produce high-quality, shelf-stable foods that meet consumer demands for safety, nutrition, and flavor.
Retort Pouches vs. Cans: A Comprehensive Comparison

The comparison between retort pouches and cans encompasses several key aspects that determine their suitability for different applications. Both packaging solutions have their unique advantages and disadvantages, which are crucial in deciding the most appropriate option for specific food products.
Structural Differences Between Pouches and Cans
Retort pouches and cans exhibit distinct structural differences that impact their functionality and user experience. Retort pouches are flexible, lightweight, and can be easily stored in compact spaces, making them ideal for outdoor activities or emergency food supplies. In contrast, cans are rigid, heavier, and require more storage space.
The flexibility of retort pouches also allows for more efficient packaging and transportation. Unlike cans, which are manufactured in specific sizes and shapes, retort pouches can be customized to fit various product volumes.
Processing Time and Energy Efficiency
Processing time and energy efficiency are critical factors in the production of shelf-stable food products. Retort pouches generally require shorter processing times compared to cans due to their thinner material and more efficient heat transfer.
This reduced processing time translates into energy savings, as less energy is required to achieve the same level of sterilization. Additionally, the lower thermal mass of retort pouches contributes to their energy efficiency.
Consumer Convenience and User-Friendliness
Consumer convenience is a significant consideration in the packaging of food products. Retort pouches offer several advantages in this regard, including easy opening mechanisms and re-sealable options, which enhance user-friendliness.
The compact nature of retort pouches also makes them more convenient for consumers to carry and store. Furthermore, the ability to heat the contents directly in the pouch adds to the convenience.
Cost Considerations: Material and Equipment
The cost implications of choosing between retort pouches and cans involve both material and equipment expenses. While the material cost of retort pouches can be lower than that of cans, the overall cost-effectiveness depends on various factors, including production volume and equipment requirements.
The initial investment in retort packaging equipment can be higher, but the long-term benefits, such as reduced energy consumption and increased production efficiency, can offset these costs.
Retort Packaging Materials: Composition and Structure
The composition and structure of retort packaging materials are vital for achieving commercial sterility in food preservation. These materials must withstand high temperatures and pressures during the retort process while maintaining their integrity and preventing contamination.
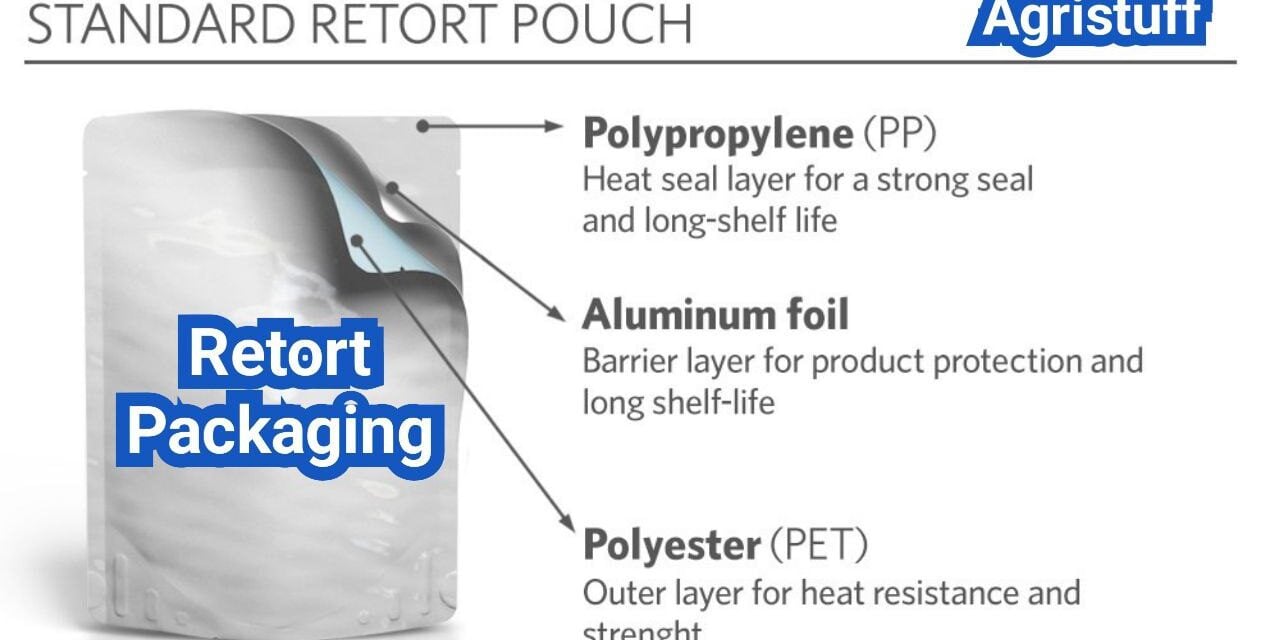
Typical Material Structure of Retort Pouches
Retort pouches are typically constructed using a multilayer structure, which provides the necessary barrier properties and strength. The most common configuration is a trilaminate structure, consisting of:
- A outer layer of PET (Polyethylene Terephthalate) for printability and durability
- A middle layer of aluminum foil for barrier properties
- An inner layer of polypropylene or other heat-sealable material for sealing
This trilaminate retort pouch construction offers excellent barrier properties against oxygen, moisture, and light, ensuring the quality and safety of the packaged food.
Trilaminate and Multilayer Material Combinations
In addition to trilaminate structures, more complex multilayer material combinations are also used in retort packaging. These may include additional layers for enhanced barrier properties, such as:
- Nylon or other high-barrier polymers
- EVOH (Ethylene-Vinyl Alcohol) for improved oxygen barrier
- Additional layers of polypropylene or polyethylene for strength and sealability
These multilayer packaging structures provide enhanced protection for sensitive products and can be tailored to specific product requirements.
Aluminum Retort Pouches vs. Transparent Retort Pouches
Retort pouches can be categorized into two main types based on their appearance: aluminum retort pouches and transparent retort pouches.
| Feature | Aluminum Retort Pouches | Transparent Retort Pouches |
|---|---|---|
| Barrier Properties | Excellent barrier against light, oxygen, and moisture | Good barrier properties, but may require additional layers |
| Product Visibility | Opaque, conceals product appearance | Transparent, allows product visibility |
| Branding and Marketing | Limited branding opportunities due to opacity | Offers opportunities for branding and product display |
Why Multilayer High Barrier Packaging Matters
Multilayer high barrier packaging is crucial for maintaining the quality and safety of retorted products. By combining different materials, manufacturers can create packaging that provides:
- Excellent barrier properties against oxygen, moisture, and light
- Strong seal integrity to prevent leakage
- Durability and resistance to puncture and abrasion
The use of high barrier packaging materials ensures that retorted products remain safe and fresh throughout their shelf life, meeting consumer expectations and regulatory requirements.
Types of Retort Packaging Solutions

Different types of retort packaging solutions cater to the diverse requirements of food manufacturers and consumers alike. The versatility in retort packaging is driven by the need for convenience, product safety, and shelf appeal. Various formats have been developed to meet these demands, each with its unique characteristics and benefits.
Stand Up Retort Pouches
Stand up retort pouches are a popular choice for packaging a wide range of food products. Their ability to stand upright on store shelves enhances product visibility and consumer convenience. These pouches are designed with a bottom gusset that allows them to stand up, making them ideal for products like soups, sauces, and ready-to-eat meals.
Key benefits: Enhanced shelf presence, consumer convenience, and flexible packaging options.
Retort Spout Pouches
Retort spout pouches combine the advantages of retort packaging with the convenience of a spout for easy dispensing. These pouches are particularly suitable for products like baby food, pet food, and beverages. The spout allows for controlled pouring and resealing, enhancing user experience.
Advantages: Convenient dispensing, resealable, and suitable for a variety of products.
Three Side Seal Retort Pouches
Three side seal retort pouches are sealed on three sides, providing a secure and leak-proof package. This format is versatile and can be used for a wide range of products, from liquid foods to solid meals. The three-side seal construction ensures strong seal integrity, crucial for maintaining product freshness.
Features: Secure sealing, leak-proof, and adaptable to different product types.
Tetra Recart Packaging
Tetra Recart packaging is a retortable carton package that offers a unique combination of convenience, sustainability, and product protection. Made from paperboard, these cartons are sterilized and filled with product in a aseptic environment. Tetra Recart is an eco-friendly option that appeals to consumers looking for sustainable packaging.
Benefits: Eco-friendly, convenient, and provides excellent product protection.
The diverse range of retort packaging solutions allows manufacturers to choose the most appropriate format for their products, balancing factors like consumer convenience, product safety, and environmental impact.
The Retort Packaging Process: Step-by-Step Guide
Retort packaging involves a meticulous process that includes filling, sealing, processing, and cooling to achieve commercial sterility. This process is crucial for ensuring the safety and quality of the final product.
Step 1: Product Filling and Headspace Management
The first step in the retort packaging process is product filling. This involves filling the retort pouches or containers with the prepared food product. It’s essential to manage the headspace effectively, as it affects the packaging’s ability to withstand the retort process. Headspace management is critical because it influences the thermal processing dynamics and the overall safety of the product.
- Ensure accurate filling levels to avoid overfilling or underfilling.
- Manage headspace to allow for expansion during thermal processing.
Step 2: Heat Sealing the Package
After filling, the next step is heat sealing the package. This involves sealing the retort pouches or cans using a heat sealer. The seal must be strong and hermetic to prevent any contamination during the retort process. Proper heat sealing techniques are vital to ensure the integrity of the package.
- Use appropriate heat sealing parameters (temperature, pressure, time).
- Verify seal integrity through regular testing.
Step 3: Retort Processing and Temperature Control
Retort processing is the heart of the packaging process, where the sealed packages are subjected to high temperatures and pressures to achieve commercial sterility. Temperature control is paramount during this step to ensure that the product is heated uniformly and that the target temperature is achieved.
- Monitor temperature and pressure throughout the retort process.
- Ensure uniform heating to prevent underprocessing.
Step 4: Container Cooling and Water Sanitation
After retort processing, the packages are cooled to stop the cooking process and to prepare them for storage or distribution. Container cooling must be done carefully to prevent contamination. Water sanitation is also critical during this step to prevent post-process contamination.
- Use chlorinated water for cooling to prevent contamination.
- Monitor water quality and sanitation regularly.
By following these steps meticulously, manufacturers can ensure that their retort-packaged products are safe, of high quality, and compliant with regulatory requirements.
Retort Packaging in the U.S.: Regulatory Framework

The U.S. retort packaging industry is subject to a stringent regulatory framework to ensure food safety. This framework is designed to protect consumers from potential health risks associated with improperly processed or contaminated food products.
Food Canning Establishment (FCE) Registration Requirements
All establishments involved in the processing, manufacturing, or packing of canned foods must register with the FDA as a Food Canning Establishment (FCE). This registration is a critical step in ensuring compliance with federal regulations. To register, establishments must provide detailed information about their operations, including the types of products they process and their processing procedures.
The FCE registration process involves submitting FDA Form 2541-a, which requires information about the establishment, including its name, address, and the types of products it handles. This registration must be renewed annually and updated whenever there are significant changes in the establishment’s operations.
Low Acid Canned Foods (LACF) Regulations
Low Acid Canned Foods (LACF) regulations are a crucial component of the regulatory framework governing retort packaging. These regulations are designed to prevent Clostridium botulinum contamination, a potentially deadly form of food poisoning. LACF regulations require that all low-acid canned foods be processed according to a scheduled process that has been filed with the FDA.
The scheduled process must be developed by a process authority, an individual or organization with the expertise to design and validate thermal processes for canned foods. The process authority must ensure that the scheduled process achieves the required level of commercial sterility.
Scheduled Process Filing FDA Form 2541
Filing a scheduled process with the FDA involves submitting FDA Form 2541, which provides detailed information about the processing procedures, including temperature, time, and other critical parameters. This form must be filed for each product and container size, and it must be accompanied by documentation from the process authority validating the scheduled process.
The scheduled process filing is a critical step in ensuring compliance with LACF regulations. It provides the FDA with the necessary information to verify that the processing establishment is operating according to approved procedures.
Process Authority Retort Validation
Process authority retort validation is a critical component of ensuring the safety and integrity of retort-packaged foods. The process authority must validate the retort process to ensure that it achieves the required level of commercial sterility. This involves conducting thorough analyses and tests to confirm that the processing equipment and procedures are adequate.
Retort validation typically involves a series of tests, including temperature distribution studies and microbiological challenge tests. These tests help to ensure that the retort is functioning correctly and that the scheduled process is effective in achieving commercial sterility.
Pouch Seal Integrity Testing Methods
To guarantee the safety and quality of food packaged in retort pouches, rigorous seal integrity testing is necessary. Seal integrity is critical because it prevents contamination and ensures the shelf stability of the product.
ASTM F88 Seal Strength Test
The ASTM F88 test is a widely recognized method for evaluating the seal strength of pouches. This test involves peeling the seal at a controlled rate to measure its strength. Seal strength is a crucial parameter as it directly affects the package’s ability to withstand handling and transportation stresses.
ASTM D3078 Bubble Emission Leak Test
The ASTM D3078 test, also known as the bubble emission test, is used to detect leaks in the pouch seal. The package is submerged in a liquid, and air is forced out of the package. The presence of bubbles indicates a leak, helping to identify potential seal integrity issues.
Dye Penetration Package Integrity Test
The dye penetration test involves applying a colored dye to the seal area. If there’s a leak, the dye penetrates the seal, making it visible. This method is effective for detecting even small leaks that could compromise the package’s integrity.
Visual Inspection Protocols
Visual inspection is a fundamental aspect of quality control. Trained inspectors examine the pouches for visible signs of seal defects, such as wrinkles, creases, or improper seal formation. While not as precise as other methods, visual inspection can be an effective first line of defense against seal integrity issues.
Implementing a combination of these testing methods can provide comprehensive assurance of pouch seal integrity. By doing so, manufacturers can ensure their products remain safe and of high quality throughout their shelf life.
Quality Control: Double Seam Inspection and Can Defects
Double seam inspection and can defect detection are crucial quality control measures in the production of retort packaging. Ensuring the integrity of the double seam is vital for preventing leakage and contamination.
Critical Double Seam Measurements
To ensure the quality of the double seam, several critical measurements must be taken. These include:
- Overlap: The amount of can flange that overlaps with the cover hook.
- Tightness: Measured by the tightness rating or visually assessed.
- Thickness: The total thickness of the double seam.
- Length: The length of the double seam.
These measurements are essential for determining the integrity of the seam and ensuring that it meets the required standards.
Common Can Seam Defects
Several defects can occur during the double seaming process, including:
- Droop: A condition where the double seam droops or sags.
- False Seam: A seam that appears correct but lacks proper overlap.
- Cut Over: A condition where the can flange is cut during the seaming process.
Identifying and addressing these defects is crucial for maintaining the quality and safety of the packaged product.
Inspection Equipment and Frequency
Various inspection equipment is used to monitor the double seam quality, including:
- Manual micrometers for measuring seam dimensions.
- Seam inspection machines for automated measurement and analysis.
Regular inspection is necessary to catch any defects or irregularities in the double seam. The frequency of inspection depends on the production volume and the specific requirements of the packaging line.
Container Cooling Water Sanitation and Post-Process Safety
Ensuring the sterility of container cooling water is crucial for preventing post-process contamination in retort packaging. After the retort process, containers are typically cooled using water to reduce their temperature. If this cooling water is not properly sanitized, it can become a source of contamination.
Chlorination Requirements for Cooling Water
One of the most effective methods for sanitizing cooling water is through chlorination. The chlorination requirements for cooling water are stringent to ensure that any potential bacteria or microorganisms are eliminated. Typically, a free chlorine level of at least 2 ppm is recommended at the water discharge point.
- Regular monitoring of chlorine levels is essential.
- pH levels of the water should be maintained between 6.5 and 7.5 for optimal chlorination effectiveness.
- Chlorine levels should be checked at regular intervals, and records should be maintained.
Preventing Post-Process Contamination
Post-process contamination is a significant risk in retort packaging, potentially leading to product spoilage and safety issues. To mitigate this risk, several measures can be implemented:
- Using sanitized cooling water that meets or exceeds potable water standards.
- Maintaining a positive water pressure to prevent any potential backflow into the containers.
- Ensuring that the cooling water system is designed and operated to prevent contamination.
Water Quality Monitoring Procedures
Effective water quality monitoring is critical for ensuring the safety of the cooling water. This involves regular testing for various parameters, including:
| Parameter | Acceptable Range | Frequency of Testing |
|---|---|---|
| pH | 6.5 – 7.5 | Daily |
| Free Chlorine | ≥ 2 ppm | Every 4 hours |
| Microbiological Count | < 1 CFU/mL | Weekly |
By implementing these measures and maintaining rigorous water quality monitoring procedures, manufacturers can significantly reduce the risk of post-process contamination and ensure the overall safety and quality of their retort-packaged products.
Thermal Process Deviation Handling
Thermal process deviations can lead to underprocessing, resulting in potential food safety issues. Effective handling of these deviations is crucial to ensure the sterility and safety of the food products.
Identifying Process Deviations
Process deviations occur when the actual retort process does not follow the scheduled process. Identifying these deviations promptly is critical for taking corrective actions. Common indicators include:
- Temperature control failures
- Incorrect processing times
- Equipment malfunctions
Immediate Response Protocols
Upon identifying a process deviation, immediate action is required to mitigate potential risks. This includes:
- Stopping the retort process
- Assessing the deviation’s impact on product safety
- Notifying relevant personnel and regulatory bodies if necessary
Product Disposition and Reprocessing Decisions
Deciding the fate of products affected by process deviations involves careful consideration. Factors include:
- The nature and extent of the deviation
- The potential risk to consumer safety
- The feasibility of reprocessing
Reprocessing may be an option if the deviation can be corrected and the product brought within safe parameters. Otherwise, product disposal may be necessary.
Documentation and FDA Notification
Thorough documentation of process deviations, including the deviation details, response actions, and product disposition, is essential. In cases where the deviation affects product safety, FDA notification may be required. This ensures compliance with regulatory requirements and maintains transparency.
Shelf-Stable Food Packaging Failures: Real-World Examples

The importance of reliable packaging for shelf-stable foods cannot be overstated, as failures can have serious consequences. Shelf-stable food packaging failures, although rare, can lead to product spoilage, financial losses, and damage to brand reputation. Understanding the causes and consequences of these failures is crucial for manufacturers to implement effective preventive measures.
Seal Failure Causes and Consequences
Seal failure is a critical issue in shelf-stable food packaging, potentially leading to contamination and spoilage. Common causes include inadequate sealing temperature, insufficient pressure, or improper sealing time. For instance, a well-documented case involved a major food manufacturer who experienced a significant recall due to seal failures in their retort pouches, resulting in consumer complaints about leakage and spoilage.
Consequences of Seal Failure:
- Product contamination and spoilage
- Financial losses due to recalls and product replacement
- Damage to brand reputation and consumer trust
Underprocessing and Spoilage Issues
Underprocessing occurs when the product is not subjected to sufficient heat or processing time, leading to potential spoilage. This can happen due to equipment malfunction, incorrect process settings, or human error. A notable example is the recall of canned vegetables due to underprocessing, which led to reports of spoilage and consumer illness.
Critical Factors in Underprocessing:
- Inadequate temperature control during processing
- Insufficient processing time
- Equipment failure or malfunction
Material Delamination Problems
Material delamination refers to the separation of layers within the packaging material, potentially compromising the barrier properties and leading to contamination. This issue can arise from improper material selection, manufacturing defects, or external factors like temperature fluctuations. For example, a case study on retort pouch delamination highlighted the importance of material compatibility and manufacturing quality control.
Lessons from Industry Recalls
Industry recalls due to packaging failures provide valuable lessons for manufacturers. Analyzing these cases can help identify common causes and preventive measures. A review of recent recalls reveals that seal failures and underprocessing are among the top reasons for packaging-related recalls in the shelf-stable food sector.
| Cause of Failure | Consequence | Preventive Measure |
|---|---|---|
| Seal Failure | Product Contamination, Spoilage | Regular Seal Integrity Testing, Proper Sealing Techniques |
| Underprocessing | Spoilage, Consumer Illness | Correct Process Settings, Equipment Maintenance |
| Material Delamination | Compromised Barrier Properties | Material Selection, Quality Control |
Retort Packaging Machine: Types and Price Considerations

When it comes to retort packaging, the machinery used can significantly impact the efficiency and quality of the final product. The right retort packaging machine is crucial for achieving commercial sterility while maintaining product integrity.
Batch Retort Systems
Batch retort systems are designed for flexibility and are often used in smaller-scale operations or for products that require special processing conditions. These systems process products in batches, allowing for easier handling of diverse product lines.
Advantages of batch retort systems include:
- Flexibility in processing different product types
- Easier maintenance and cleaning
- Lower initial investment compared to continuous systems
Continuous Retort Systems
Continuous retort systems are ideal for high-volume production, offering efficiency and consistency. These systems process products continuously, reducing labor costs and increasing throughput.
Key benefits of continuous retort systems:
- High production capacity
- Consistent processing conditions
- Reduced labor costs
Retort Packaging Machine Price Ranges
The cost of retort packaging machines varies widely based on the type, size, and features. Batch retort systems can range from $50,000 to $500,000, while continuous systems can cost between $200,000 and $1 million or more.
Factors Affecting Equipment Investment
Several factors influence the investment in retort packaging machinery, including production volume, product type, and desired level of automation. Additionally, considerations such as energy efficiency, maintenance costs, and compatibility with existing production lines play a significant role in equipment selection.
Retort Packaging Examples and Applications

From convenience foods to military MREs, retort packaging plays a crucial role in preserving the quality of products. This packaging technology has found applications across various industries, including food, military, and pet care.
Ready-to-Eat Meal Market
The ready-to-eat meal market has seen significant growth, with retort packaging being a key enabler. Brands like Chef Boyardee and Hormel use retort pouches for their convenience foods, offering consumers easy-to-prepare meals.
Military MRE Retort Pouches
Military MRE (Meals, Ready-to-Eat) retort pouches are designed to withstand harsh conditions. These pouches are used to package a variety of meals, including beef stew and chicken fajitas, providing sustenance for soldiers in the field.
Pet Food Retort Packaging
The pet food industry has also adopted retort packaging for its ability to preserve nutritional value. Brands like Merrick and The Honest Kitchen use retort pouches for their pet food products, offering a range of flavors and textures.
Soups, Sauces, and Liquid Products
Retort packaging is not limited to solid foods; it’s also used for soups, sauces, and other liquid products. Companies like Campbell’s use retort pouches for their soups, providing a convenient and shelf-stable product.
| Industry | Product Examples | Benefits of Retort Packaging |
|---|---|---|
| Ready-to-Eat Meals | Chef Boyardee, Hormel | Convenience, Shelf-Stability |
| Military MREs | Beef Stew, Chicken Fajitas | Durability, Nutritional Preservation |
| Pet Food | Merrick, The Honest Kitchen | Nutritional Preservation, Variety |
| Soups and Sauces | Campbell’s | Convenience, Shelf-Stability |
Innovations and Future Trends in Retort Packaging
Innovations in retort packaging are transforming the way food is preserved, packaged, and consumed. The industry is witnessing significant advancements in material science, consumer convenience, and sustainability.
Recyclable Retort Pouch Development
The development of recyclable retort pouches is a significant trend in the industry. Manufacturers are working towards creating pouches that are not only functional but also environmentally friendly. Recyclable materials are being integrated into retort pouch construction to reduce waste and improve sustainability.
Transparent and Clear Retort Pouch Technology
Transparent retort pouches are gaining popularity as they offer consumers a clear view of the product. This trend is driven by consumer preference for visibility and the desire to showcase the product’s quality. Advanced barrier materials are being used to maintain the pouch’s transparency while ensuring the product’s safety and shelf life.
Easy-Open Features and Consumer Convenience
Easy-open features are becoming a standard in retort packaging, enhancing consumer convenience. Manufacturers are incorporating easy-tear notches and other innovative opening mechanisms to make the product more user-friendly.
Sustainable Material Alternatives
The shift towards sustainable material alternatives is a key trend in retort packaging. Bioplastics, recyclable materials, and reduced packaging are some of the strategies being adopted to minimize environmental impact. Companies are exploring biodegradable materials and monomaterial constructions to improve recyclability.
| Innovation | Description | Benefit |
|---|---|---|
| Recyclable Retort Pouches | Made from materials that can be recycled | Reduces environmental waste |
| Transparent Retort Pouches | Allows consumers to see the product | Enhances consumer trust and satisfaction |
| Easy-Open Features | Incorporates easy-tear notches or mechanisms | Improves consumer convenience |
| Sustainable Materials | Uses bioplastics or recyclable materials | Reduces environmental impact |
At The End of: Retort Packaging
The world of retort packaging has evolved significantly, driven by advancements in technology and changing consumer demands. As discussed, retort packaging plays a crucial role in food preservation, offering a reliable method for achieving commercial sterility in various products.
The comparison between retort pouches and cans highlights the benefits of each, including processing time, energy efficiency, and consumer convenience. The materials used in retort packaging, such as trilaminate and multilayer combinations, provide essential barrier properties that preserve product quality.
Regulatory compliance, quality control measures, and innovations in retort packaging are shaping the industry’s future. The development of recyclable retort pouches, transparent packaging, and easy-open features are enhancing consumer experience while addressing environmental concerns.
As the demand for shelf-stable foods continues to grow, the retort packaging industry is poised for further innovation. The future of retort packaging looks promising, with potential advancements in sustainable materials and packaging design. In conclusion, retort packaging remains a vital component of the food packaging industry, offering a reliable and efficient solution for preserving a wide range of products.
FAQ
What is retort packaging?
Retort packaging is a method of food preservation that involves sterilizing food products in a sealed container through high-temperature processing, resulting in commercially sterile and shelf-stable products.
How does retort packaging achieve commercial sterility?
Retort packaging achieves commercial sterility through a high-temperature sterilization process that eliminates harmful microorganisms, ensuring the product remains safe for consumption over an extended period.
What are the advantages of retort pouches over cans?
Retort pouches offer several advantages, including faster processing times, improved energy efficiency, enhanced consumer convenience, and potentially lower material costs compared to traditional cans.
What materials are used in retort packaging?
Retort packaging typically involves multilayer materials, including combinations of plastic, aluminum foil, and paper, which provide barrier properties, strength, and heat resistance necessary for the sterilization process.
How is seal integrity tested in retort pouches?
Seal integrity in retort pouches is tested using various methods, including ASTM F88 seal strength tests, ASTM D3078 bubble emission leak tests, and dye penetration tests to ensure the seal is leak-tight and maintains package integrity.
What are the regulatory requirements for retort packaging in the U.S.?
Retort packaging in the U.S. is subject to regulations such as Food Canning Establishment (FCE) registration, Low Acid Canned Foods (LACF) regulations, and scheduled process filing with the FDA to ensure compliance with food safety standards.
What is the process for handling thermal process deviations?
Handling thermal process deviations involves identifying the deviation, implementing immediate response protocols, determining product disposition, and notifying the FDA as required, to ensure that affected products are handled safely and in compliance with regulations.
What are some common failures in shelf-stable food packaging?
Common failures include seal failures, underprocessing, and material delamination, which can lead to product spoilage, contamination, or other safety issues, highlighting the importance of robust quality control measures.
What types of retort packaging machines are available?
Retort packaging machines include batch and continuous systems, with varying capacities and features, and prices that depend on factors such as production volume, automation level, and specific application requirements.
What are the future trends in retort packaging?
Future trends in retort packaging include the development of recyclable and transparent pouches, easy-open features, and the use of sustainable materials, driven by consumer preferences, environmental concerns, and advancements in packaging technology.
What are the benefits of using retort packaging for ready-to-eat meals?
Retort packaging offers benefits for ready-to-eat meals, including convenience, long shelf life, and the ability to preserve nutritional value and flavor, making it a popular choice for military, outdoor, and consumer markets.
How does retort packaging preserve nutrition and flavor?
Retort packaging preserves nutrition and flavor through a controlled sterilization process that minimizes nutrient degradation and flavor loss, while also preventing contamination and spoilage.
Is Retort Packaging safe at room temperature?
Yes, when the product is properly processed to commercial sterility and the container remains hermetically sealed, but safety depends on validated controls and integrity over time. If integrity is compromised, the risk profile changes and you need clear evaluation and disposition rules rather than assumptions.
Do I need to register and file processes for Retort Packaging?
Many shelf-stable low-acid canned and acidified foods require establishment registration and scheduled process filing tied to each product and container/process combination. The safest approach is to treat filings and change control as part of product development so packaging changes don’t create compliance surprises. FDA instructions for electronic submission of AF/LACF forms
What if my product is acidified rather than low-acid?
Retort Packaging can still be used, but acidified foods have their own processes and controls, and the critical factors differ from low-acid systems. In practice, you still need disciplined pH control, verification, and documentation, plus packaging integrity that prevents post-process contamination. 21 CFR Part 114 (acidified foods controls)
Is Retort Packaging basically the same as home pressure canning?
The core idea of using pressure and heat is related, but commercial systems use validated scheduled processes, controlled equipment, and documented records at a scale and precision that home methods don’t replicate. If you are converting a “home recipe” into a commercial shelf-stable product, treat it as a new technical project with process authority involvement rather than a simple scale-up. CDC botulism prevention guidance related to canning risks
Conclusion of Retort Packaging: Pouches vs. Cans
Shelf-stable foods look simple on the outside, but the safety margin comes from engineering, controls, and verification—and Retort Packaging is where those pieces meet in the real world for soups, sauces, ready meals, and more. If you’re building a U.S. product line (or advising a farm brand that wants room-temperature distribution), your packaging choice directly affects heat penetration, seal risk, and how you prove compliance. This guide focuses on practical decisions and the failure patterns that actually show up in plants and warehouses. FDA LACF and acidified foods regulatory hub
What it is (and what it is not)
At its core, Retort Packaging means a hermetically sealed container that is thermally processed in a pressure vessel to achieve commercial sterility, so the product can be stored safely at ambient temperatures. The “retort” part is the controlled combination of time, temperature, and pressure; the “packaging” part is the container system that must survive the process and keep microbes out afterward. When either side is weak, shelf stability becomes a gamble instead of a validated outcome. Purdue Extension overview of retort processing technology
A common confusion is treating Retort Packaging as a marketing label rather than a process outcome, but regulators and process authorities care about measurable controls like minimum initial temperature, venting, process time, and cooling conditions. “Shelf-stable” is not a vibe—it’s the documented result of a scheduled process matched to a specific product, container type, and size. That’s why the same recipe can be safe in one container and risky in another. Codex hygienic practice for low-acid and acidified low-acid canned foods
Retort vs. aseptic: don’t confuse the process
Retort Packaging is often mixed up with aseptic packaging, but they’re different workflows with different failure modes and validation expectations. Aseptic systems sterilize product and package separately, then fill and seal in a controlled sterile environment; retort systems seal first and sterilize the sealed container in the retort. Operationally, that changes what you monitor, how you investigate deviations, and which container defects matter most. USDA explanation of retort vs aseptic packaging
U.S. compliance basics (FDA-focused)
In the U.S., Retort Packaging for low-acid canned foods and acidified foods typically triggers facility registration and scheduled process filing expectations, and those filings are tied to exact product/container/process combinations. The fastest way to get into trouble is assuming a “one-time filing” covers every size, style, or packaging format, because even small changes can affect heat penetration and critical factors. Treat compliance as a design input, not an afterthought. FDA establishment registration and process filing overview
Retort Packaging also lives alongside broader food safety programs, so your thermal process controls don’t replace sanitation, allergen management, supplier controls, or preventive controls where applicable. A strong operation aligns its thermal critical factors with the rest of the food safety plan, so investigations and corrective actions don’t conflict across teams. This is especially important when you co-pack or run multiple products through shared systems. FDA guidance on LACF regulation and related expectations
Why the safety margin is unforgiving
The reason Retort Packaging is regulated so tightly is simple: low-acid, low-oxygen foods can support hazards that do not “announce themselves” with smell or visible spoilage. The scheduled process is designed to control organisms of public health concern, and the package must prevent recontamination after processing. If you’re missing data, relying on tribal knowledge, or “eyeballing” closure quality, you’re shrinking the safety margin without realizing it.
Retort Packaging becomes especially sensitive when a closure leak allows cooling water or post-process contamination to enter, because the product environment can be ideal for toxin production if controls fail. That’s why plant operators obsess over container handling, cooling-water sanitation, and closure integrity—not because it’s paperwork, but because small defects can create big consequences. When you build your system around defect prevention, you spend less time reacting to “mystery spoilage” later. CDC overview of botulism conditions and risk factors
Pouches vs. cans: how the container changes the process
Choosing between pouches and cans in Retort Packaging is not just a cost or branding decision; it changes heat transfer, stacking patterns, abuse resistance, and your inspection approach. The “best” format is the one that matches your product properties (viscosity, particulates, fill weight), your distribution realities (e-commerce vs pallets, hot climates, long-haul), and your ability to control closure quality at speed. Start with what you can control reliably every day, not what looks best in a mockup. FDA inspection guidance focus on containers and closures
For many brands, pouches win in Retort Packaging because they’re light, space-efficient, and can heat more quickly due to thin product layers, which can help texture and flavor when the process is optimized. The tradeoff is that flexible laminates and seals can be more sensitive to abrasion, sharp particulates at the seal area, and seal-jaw variability. Pouches reward disciplined setup, tight in-process checks, and distribution testing. Open-access review on retort processing and packaged foods
Cans often win in Retort Packaging when you need maximum abuse resistance, predictable stacking, and strong protection against puncture or flex-cracking during distribution. The tradeoff is weight, slower heating in some geometries, and a closure system (double seam) that demands specific tooling, seam teardown measurements, and ongoing control of can/end compatibility. Cans also require attention to dents, corrosion, and coating compatibility over long shelf lives.
From a product-quality standpoint, Retort Packaging favors containers that minimize the “cold spot” challenge for your specific product style, because that cold spot drives the process time and therefore the heat exposure of the rest of the product. Thick purees, chunky soups, and starch-heavy meals behave differently than broths or sauces, and packaging geometry changes the heat penetration pathway. Your process authority will look at product formulation, fill method, and container orientation—not just the label claim. FDA inspection guidance on process establishment considerations
Retort systems: what your package must survive
The retort system you run can reshape what “safe” and “robust” means for Retort Packaging, because steam, water spray, water immersion, and agitating retorts expose packages to different mechanical stresses. A pouch that seals well in a lab may delaminate or develop seal wrinkles under agitation if the laminate or sealing window is marginal. Likewise, a can seam that looks fine visually may fail under real line conditions if setup, lubrication, or can/end specs drift. FDA inspection guidance on retort systems and key checks
Instrumentation is not optional in Retort Packaging because time-temperature records are how you prove you hit the scheduled process, and they’re also how you investigate deviations without guessing. That includes calibrated temperature-indicating devices, accurate recording systems, and procedures for handling emergency stops or drops in retort temperature. If your data can’t explain what happened, you’ll struggle to justify product disposition decisions. FDA inspection technical guides (instrumentation and process basics)
Materials: barriers, food-contact rules, and fit-for-process design
Materials selection for Retort Packaging should start with a simple question: can you document that every layer is suitable for food contact at the time-temperature conditions you will use? That includes laminate films, inks, adhesives, and sealants, plus can coatings and gasket materials where relevant. Good suppliers support you with declarations, specs, and change-control notices so the process authority isn’t blindsided later by a “minor” material switch.
Many pouch structures for Retort Packaging rely on high-temperature laminates engineered to survive heat, pressure, and flex without losing barrier integrity. Whether you use foil-based laminates or high-barrier polymers, you’re managing tradeoffs between oxygen barrier, puncture resistance, flex durability, and recyclability goals. The right structure is the one that stays intact through your specific retort cycle and distribution pathway, not the one with the most impressive spec sheet. 21 CFR 177.1390 (laminates and coatings for food contact)
A common outer layer in Retort Packaging is PET because it provides toughness, printability, and dimensional stability, but it’s only part of a full laminate system. PET performance also depends on adhesive systems, ink systems, and how the laminate is cured and stored before use. When you see retort “scuffing” or layer separation, the root cause is often the system—not the single material named on the datasheet. 21 CFR 177.1630 (polyethylene phthalate polymers)
The sealant layer is where Retort Packaging lives or dies, because even perfect barrier layers don’t help if the seal has channels, wrinkles, or contamination. Polypropylene is common in retortable sealants, but performance is tied to sealing temperature, dwell time, pressure, jaw condition, and how product residues behave at the seal area. A process window that is “barely acceptable” at startup often becomes unacceptable at speed, during shift changes, or when ambient conditions drift. 21 CFR 177.1520 (olefin polymers, including polypropylene)
For metal formats, corrosion control is central to Retort Packaging because a can is both a container and a long-term protective system, and the lining/coating is what separates product chemistry from the metal. Acidic or sulfur-containing foods, salt levels, and headspace conditions can stress coatings over time, and thermal processing adds another stress cycle. The practical approach is compatibility testing and supplier documentation, not assumptions based on “similar products.” 21 CFR 175.300 (resinous and polymeric coatings)
Conversations about BPA sometimes show up during Retort Packaging material reviews, especially when brands consider can linings and consumer perception. The operational takeaway is to document coating type, supplier compliance statements, and any change-control notices, and then focus on whether the system meets performance and regulatory requirements for the intended use. Don’t let myths replace documentation and fit-for-purpose testing. FDA information on BPA use in food-contact applications
Seal integrity: what “hermetic” looks like in practice
Most quality incidents in Retort Packaging trace back to closure integrity, and flexible pouches add defect patterns that can be easy to miss without structured checks. Typical pouch issues include channel leaks from contamination, wrinkles at the seal, incomplete fusion, seal creep, pinholes, and abrasion damage that develops in distribution. The best prevention is designing your fill, seal, and handling steps so defects are hard to create in the first place.
One underrated cause of failures in Retort Packaging is seal-area contamination that looks harmless during production but becomes a leak path after thermal cycling and handling. Viscous sauces, oils, starches, and small particulates can migrate into the seal zone if filling, wiping, or headspace control is inconsistent. Practical controls include better splash control at fill, tighter seal-area cleaning, and line settings that are locked with documented checks. 21 CFR Part 113 (LACF processing and control requirements)
How to test integrity (and what each test is really telling you)
A smart integrity program for Retort Packaging uses multiple methods because no single test catches every defect type, and you want early warning before defects become complaints. Visual checks, seal appearance trends, teardown inspections, and targeted destructive tests help you detect drift before it becomes a deviation. The key is to define sampling frequency, acceptance criteria, and actions that actually stop recurrence.
Seal strength testing matters in Retort Packaging because a seal that “looks fine” can still be weak, inconsistent, or sensitive to handling and thermal stress. A peel or tensile test lets you track process stability over time and compare shifts, lots, or jaw setups. Treat it like process control data, not a pass/fail ritual. ASTM F88/F88M (seal strength testing overview)
Gross leak tests support Retort Packaging by quickly finding obvious holes, major channel leaks, or damaged areas that will not survive distribution. Bubble emission methods are widely used because they’re fast and visual when paired with a vacuum chamber, and they can be effective as an in-plant screen. The limitation is that “no bubbles” doesn’t guarantee microleak-free performance, so you still need a layered program. ASTM D3078 (bubble emission leak test overview)
Microleak methods can strengthen Retort Packaging verification when you suspect very small channels that may only open under stress, but they must be validated for your materials and defect types. Dye penetration is useful in some cases, yet it can over- or under-estimate risk depending on laminate structure and how the defect behaves. The practical takeaway is to qualify your test method with known-defect samples and tie results to real distribution performance. Peer-reviewed study discussing dye penetration and package integrity testing
Even with strong testing, Retort Packaging performs best when you design the workflow so defects are unlikely: stable sealing parameters, controlled seal cleanliness, clear handling rules, and distribution packaging that prevents abrasion and corner impacts. Integrity is a system outcome that includes pouch guides, conveyor transitions, case packing, and palletizing—not just the sealer or the seam. If you fix defects only at the end, you’ll chase symptoms forever.
Scheduled process, process authority, and filings
A scheduled process is the backbone of Retort Packaging because it defines the minimum conditions needed for safety and stability for a specific product and container, including critical factors that affect heat penetration. You don’t “borrow” a process from a different container size or format without technical justification, because heat transfer changes with geometry and fill style. A qualified process authority is the guardrail that keeps optimism from becoming risk. FDA inspection guidance on scheduled process information
When you file processes for Retort Packaging, regulators expect the filing to match the real operation—equipment type, times/temperatures, initial temperature, container specs, and critical factors. If you change a pouch structure, sealing conditions, fill weight, or retort system, you should treat it as a technical change that may require re-evaluation. The best habit is change control that starts before procurement orders are placed. FDA instructions for electronic submission of AF/LACF forms
Real-world failures (what actually goes wrong)
A classic failure pattern in Retort Packaging is a temperature drop or process interruption followed by “resuming the clock” without a validated corrective action, which can make a safety evaluation impossible. Operators may treat it as a routine hiccup, but the process authority needs complete records and the right corrective steps to determine disposition. Your deviation procedure should be written, trained, and used consistently—especially during night shifts and peak production. FDA inspection guidance on temperature drops and venting issues
Another real-world issue in Retort Packaging is post-process contamination through cooling water or poor handling, especially when closure defects exist or sanitation is weak. Cooling water control is not just a “utilities” problem; it’s a food safety control that must be monitored and recorded, and it becomes more important when you run high volumes or recirculated systems. If you have unexplained spoilage, cooling water and post-process practices deserve immediate scrutiny. FDA inspection guidance on cooling water sanitation
Distribution damage is a frequent trigger for Retort Packaging complaints because dents, abrasions, and seam/edge impacts can compromise container integrity after the product leaves your facility. This is why warehouse exams and sampling plans matter: they reveal whether your packaging, case pack, and pallet patterns are actually protecting product under real freight conditions. If e-commerce is part of your plan, assume higher impacts and more abrasion unless proven otherwise. FDA inspection guidance on warehouse container examinations
Finally, Retort Packaging failures often become expensive when a plant lacks clear deviation disposition rules, so product is either wasted unnecessarily or released without defensible justification. The right system defines who evaluates deviations, what data is required, how disposition is documented, and what triggers reprocessing or destruction. If you can’t explain your decision path clearly, you’re carrying avoidable risk. FDA inspection guidance on process deviations and procedures
Retort packaging in the U.S. farm-to-market context
For farm brands, Retort Packaging can unlock room-temperature shipping and longer selling seasons for value-added products, but most farms succeed by partnering with a co-packer that already runs validated systems. The practical decision is whether your product and volume justify owning retort infrastructure, process authority services, QA testing, and compliance overhead—or whether co-manufacturing is the smarter route. If you handle meat or poultry, you also need to align with USDA/FSIS frameworks that apply to thermally processed, commercially sterile products. 9 CFR Part 431 (thermally processed, commercially sterile products)
When you move into regulated shelf-stable meats, Retort Packaging programs often include incubation and abnormal container controls, which are operationally demanding but valuable for catching issues early. Even if you’re not running those exact programs, the principle is useful: you want structured verification that your system stays stable across lots, shifts, and seasons. Many failures show up only after time, temperature cycling, and handling—so verification must match reality.
Quick checklist
Use this list to pressure-test your Retort Packaging plan before you scale runs, add SKUs, or switch packaging suppliers. It’s designed to catch the “small” gaps that often become the big, expensive failures later. FDA compliance program manual for acidified and low-acid canned foods
- Confirm product category (low-acid vs acidified) and document the basis.
- Engage a qualified process authority early (before finalizing container format and size).
- Lock container specs (pouch structure or can/end specs) with supplier change-control expectations.
- Define sealing or seaming setup parameters and create a startup verification routine.
- Map critical factors that affect heat penetration (fill weight, viscosity, particulates, headspace, orientation).
- Validate instrumentation, calibration intervals, and record review responsibilities.
- Establish an integrity testing plan (visual + strength + leak) with acceptance criteria and actions.
- Write deviation procedures for temperature drops, emergency stops, and record anomalies.
- Verify post-process handling (cooling water, container handling, drying, and storage practices).
- Run distribution testing that matches your real channels (pallet freight, parcel, hot climates).
- Build a packaging change-control trigger list (new laminate, new pouch supplier, new seamer tooling, etc.).
Common mistakes to avoid
Most Retort Packaging “surprises” are predictable patterns that show up when teams skip verification steps or assume yesterday’s settings will hold forever. Use these as prevention targets, not as a list of things to learn the hard way. FDA inspection guidance overview for LACF inspections
- Treating packaging as interchangeable: If container size or structure changes, re-evaluate heat penetration and critical factors.
- Running “too tight” seal windows: If acceptable seals require perfect conditions, you’ll fail during drift or speed changes.
- Under-controlling the seal area: Product residues at the seal zone drive channel leaks and weak seals after thermal cycling.
- Relying on a single integrity test: Pair fast screening with deeper verification tied to known-defect samples.
- Weak deviation documentation: Incomplete records make product disposition risky and often force unnecessary disposal.
- Ignoring post-process handling: Cooling water control and container handling are frequent root causes of spoilage incidents.
- Skipping distribution reality checks: Abrasion and corner impacts in transit can defeat otherwise good packages.
- No supplier change control: “Equivalent” materials can change barrier and seal performance without obvious warnings.
- Not training for shift-to-shift consistency: Sealing and seaming drift often correlates with staffing changes and handoffs.
Costs and ROI snapshot
Costs for Retort Packaging are driven less by the retort itself and more by the full system: process authority work, validation, packaging materials, QC testing, compliance administration, and distribution packaging that prevents damage. For farms and small brands, co-packing can reduce capital burden but may increase per-unit costs, minimum runs, and lead times, so the “right” model depends on volume, seasonality, and SKU complexity. A practical ROI lens is whether shelf stability unlocks higher-margin channels or reduces cold-chain costs enough to justify the added controls.
From an ROI perspective, Retort Packaging often pays back when it expands geographic reach, stabilizes inventory across harvest cycles, and lowers spoilage or returns compared with chilled distribution, but only if integrity and deviation controls are strong. Financing decisions should account for working capital (inventory and packaging), lab and testing expenses, and the time needed to validate and file changes as you expand. For brands moving from pilot to steady production, structured financing can help bridge that scale-up gap without forcing risky shortcuts. SBA 7(a) loan program overview
Final thought
Retort Packaging is most successful when you treat the container, process, and verification program as one integrated system built around prevention, not reaction. If you choose the format that matches your product physics, lock materials with change control, run a layered integrity program, and document deviations like they matter, you’ll avoid the failures that quietly erase margin and trust. FDA inspection guidance on processes and procedures
Sources & References
- FDA Guide to Inspections of Low Acid Canned Food 7
- FDA Guide to Inspections of Low Acid Canned Food 45
- FDA AF/LACF paper submissions (forms list)
- FDA instructions for paper submission of Form 2541
- FDA Draft Guidance: Chapter 16 (Acidified Foods)
- FDA instructions for electronic submission of Form 2541g (LACF aseptic systems)
- 21 CFR Part 113 (GovInfo PDF)
- 21 CFR 113.3 definitions (Cornell Law School)
- 21 CFR Part 108 (Emergency permit control)
- ASTM F1929 (dye penetration test overview)
- CDC botulism prevention guidance